Company also announces new ASCO data presentation from the prospective PROMIS study assessing the clinical impact of the definitive MammaPrint 70-gene assay results for patients previously classified as indeterminate by the 21-gene assay (OncotypeDx)
IRVINE, CA and AMSTERDAM – Agendia, Inc., a world leader in personalized medicine and molecular cancer diagnostics, announces the appointment of Gabriel N. Hortobagyi, MD, FACP, FASCO, as Chair of its Medical Advisory Board. Dr. Gabriel Hortobagyi is an internationally recognized expert in clinical and translational research of breast cancer and brings more than 35 years of experience as a Breast Medical Oncologist. He is Professor and Chair Emeritus of the Department of Breast Medical Oncology at the MD Anderson Cancer Center (MDACC) and is also the past President of the American Society of Clinical Oncology (ASCO). He is currently the Chair of the Southwest Oncology Group Breast Committee and a member of the Scientific Advisory Board of The Breast Cancer Research Foundation.
“I am thrilled for the opportunity to advise one of the leading molecular diagnostic companies in the industry,” said Dr. Hortobagyi. “Their cancer diagnostic tests, including MammaPrint and BluePrint are some of the most advanced in genomic testing field. In fact, with the recent MINDACT trial results, MammaPrint now has the highest level of evidence a breast cancer diagnostic test can achieve. The additional clinical and genomic data from that trial has the potential to significantly advance the field of breast cancer oncology even further.”
The company also announced that it will be presenting new prospective data for the MammaPrint 70-gene assay at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, which takes place on June 3-7 in Chicago. The recently completed PROMIS study (PRospective Study Of MammaPrint in Patients With an Intermediate Recurrence Score) evaluates the clinical impact of the definitive MammaPrint result in 831 ER+, LN- (lymph node negative) breast cancer patients, who were previously given an indeterminate/intermediate result with the 21-gene assay OncotypeDx (RS 18-30 clinical range, 11-25 trial range for 39-67% of tested patients).
The PROMIS poster will be presented on Sunday, June 5 from 8 a.m. to 11:30 a.m. CT. Michaela Tsai, MD, one of the four principal investigators of PROMIS, will present the study. Agendia will also be exhibiting at ASCO in Exhibit Booth #22109, from June 4-6 during Exhibit Hall hours (9 a.m. – 5 p.m.).
Session: Breast Cancer – HER2/ER
Session Title: The 70-gene signature to provide risk stratification and treatment guidance for patients classified as intermediate by the 21-gene assay
Location: Hall A, McCormick Place
Poster Session: Board #59 / Abstract #571
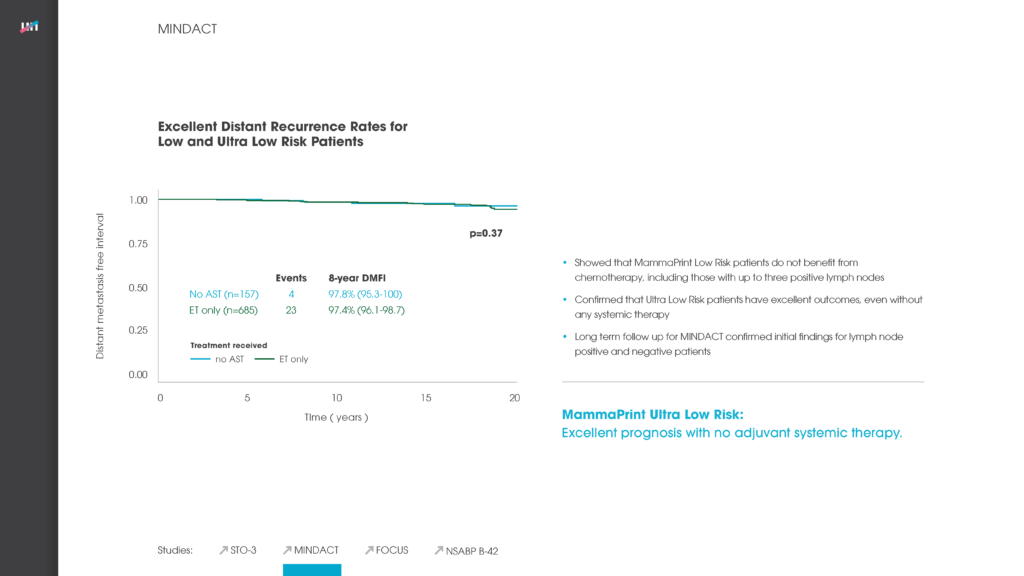
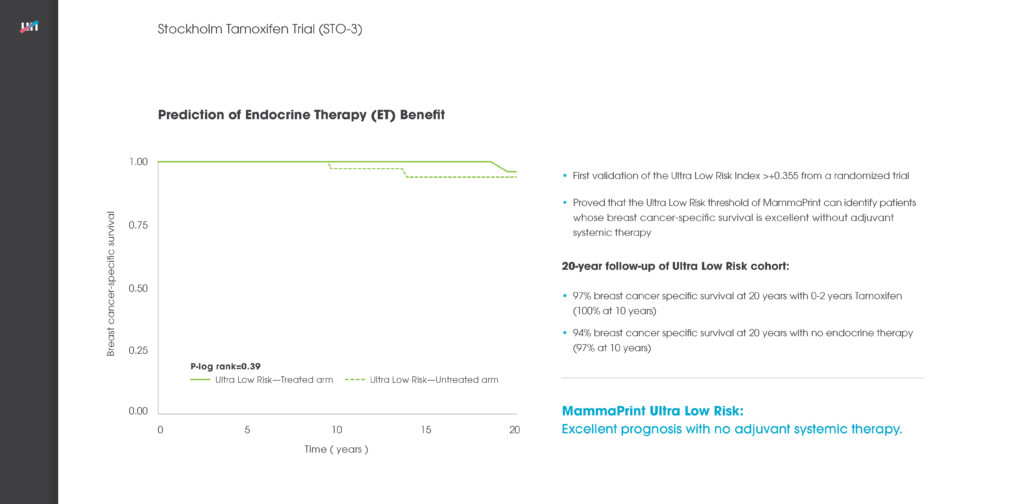
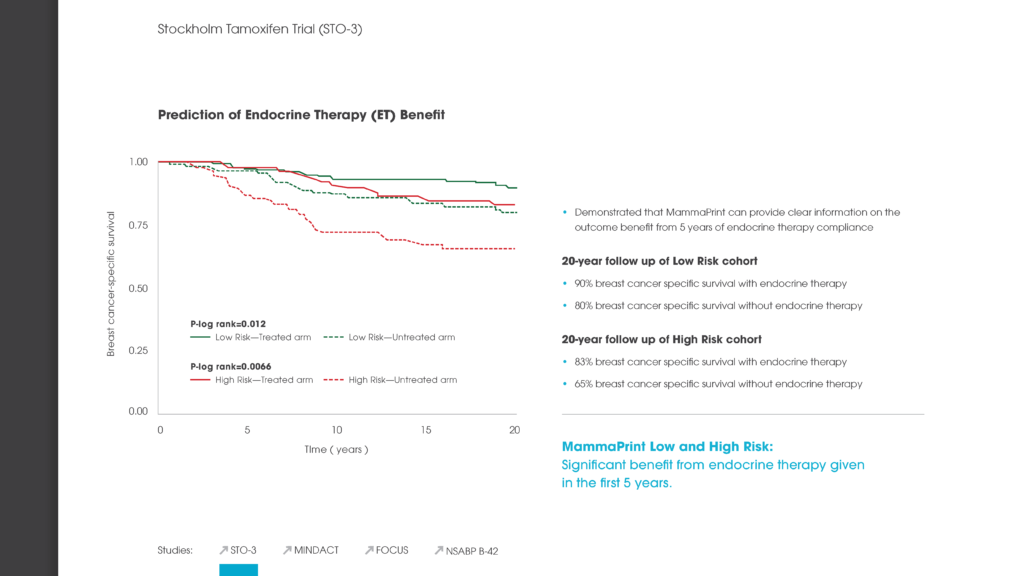
Agendia, Inc., together with the European Organisation for Research and Treatment of Cancer (EORTC) and Breast International Group (BIG), presented results from the initial analysis of the primary objective of the Microarray In Node-negative (and 1 to 3 positive lymph node) Disease may Avoid ChemoTherapy (MINDACT) study at the American Association for Cancer Research Annual Meeting 2016 in New Orleans in April 2016. As a follow-up to the primary analysis, scientific data reviews will be available at the Agendia Exhibit Booth (#22109) at ASCO as well.
The MINDACT trial is the first prospective randomized controlled clinical trial of a breast cancer recurrence genomic assay with level 1A clinical evidence and the first prospective translational research study of this magnitude in breast cancer to report the results of its primary objective.
“The positive results of the MINDACT trial along with the new PROMIS results, add further proof that the data evaluating the clinical value of MammaPrint are the most robust in the industry,” said Mark Straley, Chief Executive Officer at Agendia. “With our strong clinical trial results, our robust pipeline of products, and the recent appointments of Dr. Hortobagyi to our advisory board and Dr. Audeh as our new Chief Medical Officer, we’re making exciting advances in our continued pursuit to bring more effective, individualized treatments within reach of cancer patients.”
MINDACT included 6,693 patients and is a phase III, prospective, randomized controlled, clinical trial comparing the use of MammaPrint 70-gene assay with clinical-pathological criteria (current standard of care) for selecting early breast cancer patients who should be treated with adjuvant chemotherapy.
For more information on Dr. Hortobagyi, PROMIS or MINDACT, please visit the Agendia newsroom: http://www.agendia.com/agendia-news-and-press-releases/.
About Agendia
Agendia is a privately held, leading molecular diagnostics company that develops and markets FFPE-based genomic diagnostic products, which help support physicians with their complex treatment decisions. Agendia’s breast cancer and colorectal cancer tests were developed using an unbiased gene selection by analyzing the complete human genome. Our offerings include the FDA-cleared MammaPrint® FFPE 70-gene assay as well as the BluePrint® 80-gene molecular subtyping assay that provides deeper insight, leading to more clinically actionable biology. These tests can help physicians assess a breast cancer patient’s individual risk for metastasis – that is, which patients are more sensitive to chemo, hormonal, or combination therapy, and which patients may not require these treatments and which patients may be treated with other, less arduous and costly methods.
In addition, Agendia has a pipeline of other genomic products in development. The company collaborates with pharmaceutical companies, leading cancer centers and academic groups to develop companion diagnostic tests in the area of oncology. It is also a critical partner in the ISPY-2, NBRST and the MINDACT trials. For more information, visit www.agendia.com.
Media Contacts:
Scott Speer
FleishmanHillard
(310) 482-4283
scott.speer@fleishman.com
Léon Melens
LifeSpring Life Sciences Communication
T +31 (0)6 538 16 427
lmelens@lifespring.nl