- KANTAR survey: Only 40% of breast cancer patients know the benefits of genomic testing
IRVINE, CA and AMSTERDAM, 9 October 2017 – Breast cancer is the most feared type of cancer among women in Germany, according to a recent representative survey commissioned by Agendia, a world leader in personalized medicine and molecular cancer diagnostics. 21% of the over 1,000 women questioned in a telephone survey carried out by KANTAR Emnid said that of all cancers, they most feared breast cancer, slightly ahead of colon and lung cancer (both at 20%). Over half (57%) of respondents said that they, or a relative or friend, have already been affected by breast cancer.
The survey also highlighted a major gap in women’s knowledge about genomic testing in breast cancer. Although 73% of respondents said that they felt “rather well” or “very well” informed about breast cancer treatment options less than half (40%) knew about the role of of genomic tests to deliver important information, based on the individual biology of a breast cancer tumor, to better inform their doctors and themselves in making personalized treatment management decisions. Women need to know more about these genomic tests, such as the MammaPrint® 70-Gene Breast Cancer Risk of Recurrence Test, to ensure that they, and their family and friends are informed and empowered to request a test should they ever need it.
Current international clinical guidelines, such as those of the German Gynecological Oncology Group (AGO), American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the St. Gallen Consensus and the European Group on Tumour Markers (EGTM), recommend the use of MammaPrint and other breast cancer risk-of-recurrence genomic tests. Clinical practice guidelines provide valuable information for doctors and outline appropriate tests, methods of treatment, and care.
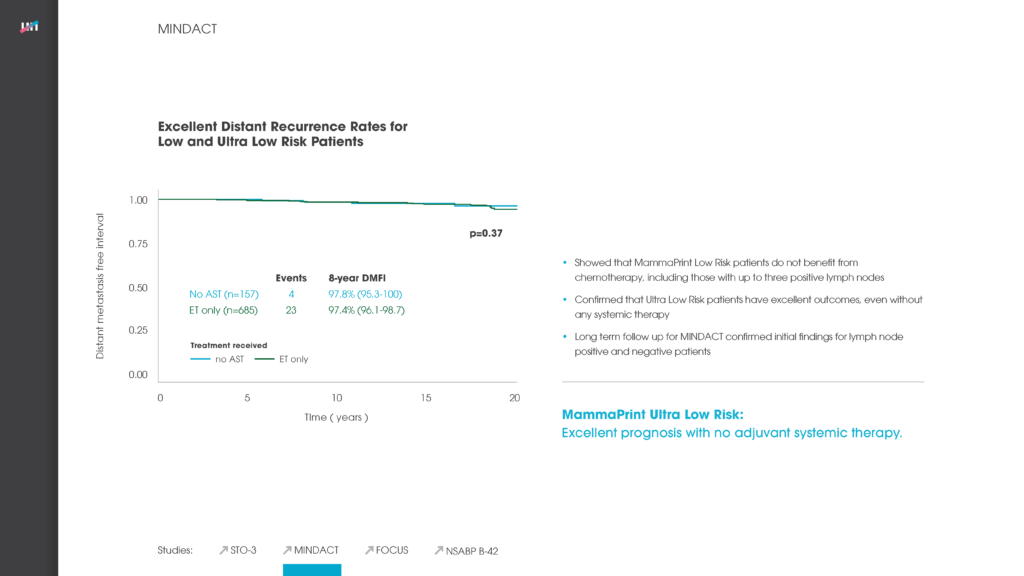
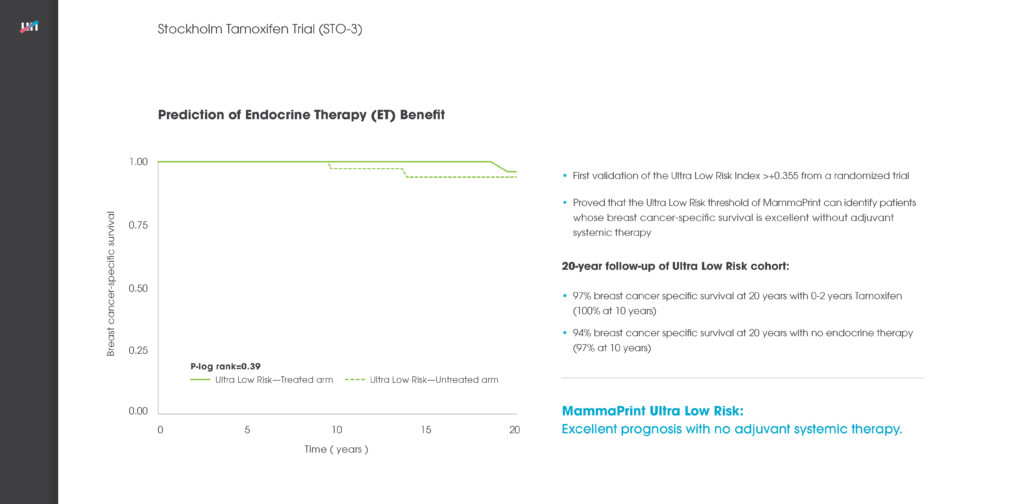
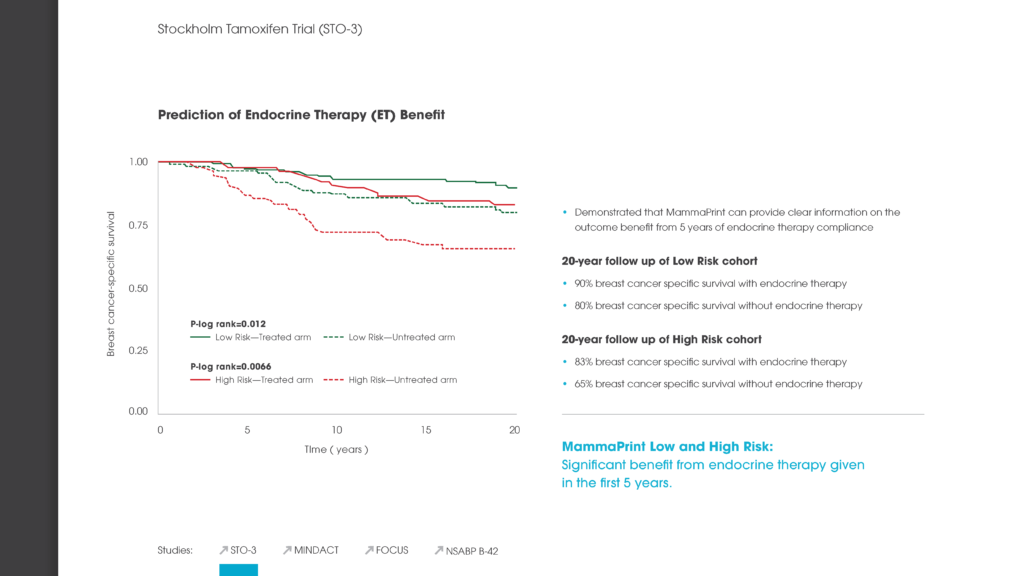
The MammaPrint test analyses the activity of the 70 genes most associated with breast cancer recurrence and provides a Low Risk or a High Risk-of-recurrence result. This enables doctors to identify those women with a certain type of early-stage invasive breast cancer who are at low risk of their breast cancer recurring and who will have no significant benefit of chemotherapy. Indeed, the findings of the MINDACT trial, published in August 2016, found that three out of four women with the most common form of breast cancer were identified by MammaPrint as Low Risk.[1]
“Every physician should consider their patient’s personal treatment preferences and ensure that they have all of the information possible on which to agree the most beneficial approach. We should explain to each appropriate breast cancer patient the opportunity that genomic testing offers and discuss all aspects of the therapy,” said breast cancer expert Prof. Dr. med. Nadia Harbeck, head of the Breast Center at the University of Munich (LMU).
“By analyzing the genes, we contribute, amongst other factors, to keeping the frequency of unnecessarily prescribed adjuvant chemotherapy as low as possible for patients with a high risk of relapse based on conventional clinicopathological factors but a low genomic risk.”
Breast cancer is the most frequently diagnosed cancer in women worldwide.[2] In 2012, there were nearly 1.7 million new breast cancer cases among women worldwide, accounting for 25% of all new cancer cases in women.[3]
– Ends –
About MammaPrint
MammaPrint is an in vitro diagnostic test, performed in a central laboratory, using the gene expression profile of breast cancer tissue samples to assess a patients’ risk for distant metastasis. MammaPrint is cleared by the US FDA and carries the CE Mark, which certifies that the test complies with the quality standards set by the European In Vitro Diagnostic Directive, enabling the use of the test in the European Union. MammaPrint is indicated for use by physicians as a prognostic marker only, along with other clinical-pathological factors. The test is not intended to determine the outcome of disease, nor to suggest or infer an individual patient’s response to therapy.
About Agendia
Agendia is a privately held, leading molecular diagnostics company that develops and markets genomic diagnostic products, which help support physicians with their complex treatment decisions. Agendia’s breast cancer tests were developed using an unbiased gene selection by analyzing the complete human genome. Our offerings include the MammaPrint® 70-Gene Breast Cancer Risk-of-Recurrence Test, and the BluePrint® Molecular Subtyping Test that provides deeper insight leading to more clinically actionable breast cancer biology.
In addition, Agendia has a pipeline of other genomic products in development. The company collaborates with pharmaceutical companies, leading cancer centers and academic groups to develop companion diagnostic tests in the area of oncology.
For more information on Agendia or the MammaPrint and BluePrint tests, you can visit Agendia’s patient site at www.KnowYourBreastCancer.com or the corporate site at www.agendia.com.
Follow Agendia, Inc. on Facebook, Twitter, or LinkedIn to keep up-to-date with the latest news.
Media Contact:
Anja Frohloff
Instinctif Partners
+49 30 2408304-18
anja.frohloff@instinctif.com
[1] Cardoso F, van’t Veer LJ, Bogaerts J et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 2016; 375: 717-29.
[2] World Health Organization. Breast cancer: prevention and control. Website. http://www.who.int/cancer/detection/breastcancer/en/index1.html Accessed March 2016.
[3] American Cancer Society. Global Cancer Facts & Figures 3rd Edition. Atlanta: American Cancer Society; 2015. (online)