- Retrospective analysis of patient samples from the prospective, randomized Stockholm Tamoxifen Trial demonstrates clinical utility of MammaPrint in identifying breast cancer patients with exceedingly low likelihood for late recurrence at 20-years
- MammaPrint Late Recurrence (20yr) Low Risk result identifies sub-group of patients with excellent survival after 20 years with little or no hormonal therapy, providing more treatment options for physicians and their patients
IRVINE, CA and AMSTERDAM – 29 June 2017 – Agendia, Inc., a world leader in personalized medicine and molecular cancer diagnostics announces new data published today in JAMA Oncology.1 Following the retrospective analysis and 20-year follow-up data from the prospective, randomized Stockholm Tamoxifen Trial, a Late Recurrence (20yr) Low Risk result (referred to as the ‘indolent’ threshold in the study) of the MammaPrint® Test has demonstrated the ability to identify a sub-group of patients with exceedingly low metastatic risk 20 years after diagnosis. These patients, of which a majority only received two years of hormonal therapy, had an observed 20-year breast cancer specific survival of 97%.
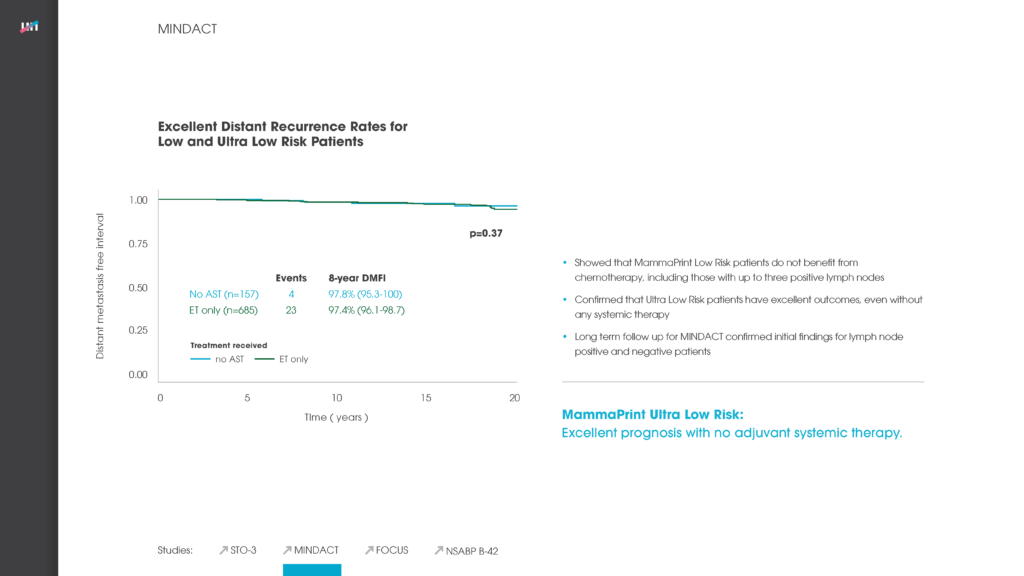
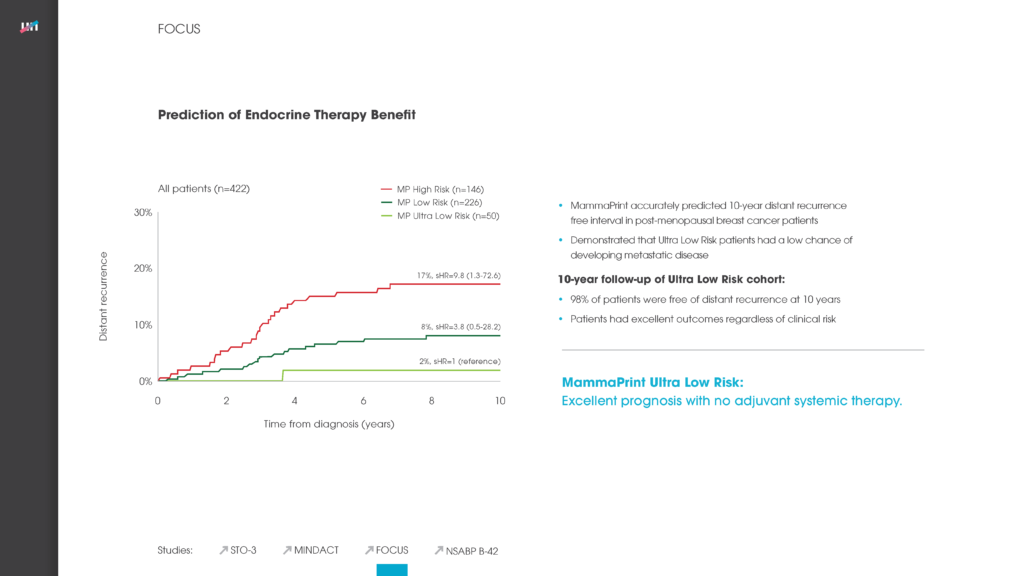
In 2016, the MINDACT trial demonstrated with the highest level of clinical evidence that MammaPrint 70-Gene Breast Cancer Risk-of-Recurrence Test can assess a patient’s risk of distant breast cancer metastasis and provides a binary High Risk or Low Risk result to help aid chemotherapy treatment decisions.2 Now, with its Late Recurrence (20yr) Low Risk result, MammaPrint is currently the only genomic signature to identify patients with indolent breast cancers who have an extremely low risk of metastatic recurrence. This information is helpful to physicians when deciding on whether to recommend extended, standard, or limit endocrine treatment for some patients.
Dr. William Audeh, Chief Medical Officer of Agendia, said: “By using MammaPrint to identify a subset of patients who have excellent breast cancer-specific survival at 20 years with limited endocrine therapy treatment post-surgery, we can provide physicians and their patients with more options for better treatment and management of the disease.
“For some women, endocrine therapy can be more difficult than chemotherapy and nearly 50% of patients are unable to complete their treatment3, so identifying those women who could do very well with limited or no treatment is important. The MammaPrint Late Recurrence (20yr) Low Risk result is clinically invaluable as these patients cannot be identified using other methods such as standard clinical-pathological factors or molecular subtyping”, said Dr. Audeh.
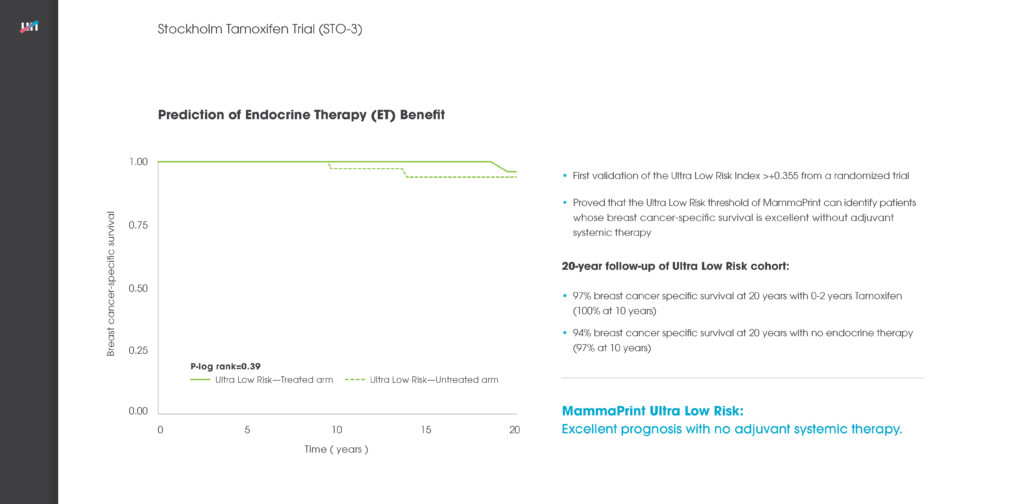
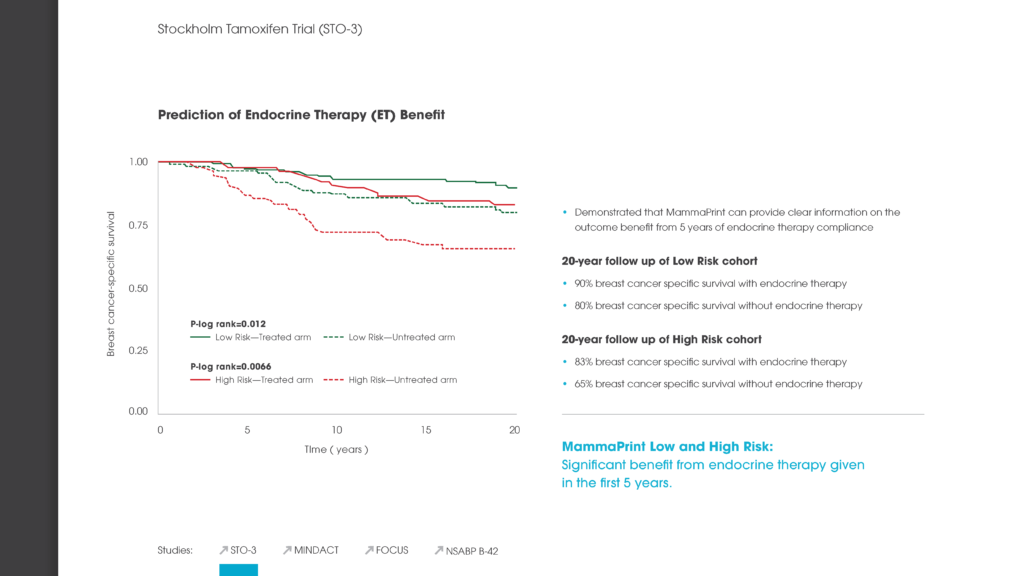
The Stockholm Tamoxifen Trial was a prospective, randomized trial conducted by the Stockholm Breast Cancer Study Group from 1976-1990. 1,780 patients were randomized to two years of tamoxifen versus no systemic therapy. Patients without a relapse after two years of tamoxifen and who re-consented were further randomized to three additional years of tamoxifen treatment, or no further therapy. Only 35% of patients received a full five-years of treatment.
For this retrospective study, 652 patient samples were assessed with MammaPrint and 58% (377) were classified as Low Risk. Of these, 26% (98) of patients were found to be MammaPrint Late Recurrence (20yr) Low Risk, indicating an excellent survival profile at 20-years.
Patients that were randomized to receive hormonal therapy with this MammaPrint Late Recurrence Low Risk result had a remarkable 97% breast cancer-specific survival 20 years after diagnosis. 65% of tamoxifen-treated patients only received it for two years. Those who received no endocrine therapy after surgery also showed an impressive 94% breast cancer specific survival rate at 20 years.
You can access the article, Molecular tools identify indolent breast cancers with ultra-low risk over two decades here: http://jamanetwork.com/journals/jamaoncology/article-abstract/2634502
1. Esserman L., et al. JAMA Oncology. doi:10.1001/jamaoncol.2017.126. Published online June 29, 2017.
2. Cardoso F, van’t Veer LJ, Bogaerts J et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 2016; 375: 717-29.
3. Chlebowski et al., Adherence to Endocrine Therapy in Breast Cancer Adjuvant and Prevention Settings. Cancer Prev Res. 2014; 7:378-387.
– Ends –
About MammaPrint
MammaPrint is an in vitro diagnostic test, performed in a central laboratory, using the gene expression profile of breast cancer tissue samples to assess a patients’ risk for distant metastasis. MammaPrint is cleared by the US FDA and carries the CE Mark which certifies that the test complies with the quality standards set by the European In Vitro Diagnostic Directive, enabling the use of the test in the European Union. MammaPrint is indicated for use by physicians as a prognostic marker only, along with other clinical-pathological factors. The test is not intended to determine the outcome of disease, nor to suggest or infer an individual patient’s response to therapy.
About Agendia
Agendia is a privately held, leading molecular diagnostics company that develops and markets genomic diagnostic products, which help support physicians with their complex treatment decisions. Agendia’s breast cancer tests were developed using an unbiased gene selection by analyzing the complete human genome. Our offerings include MammaPrint®, a 70-Gene Breast Cancer Risk-of-Recurrence test, and BluePrint®, a Molecular Subtyping Assay that provides deeper insight leading to more clinically actionable breast cancer biology.
In addition, Agendia has a pipeline of other genomic products in development. The company collaborates with pharmaceutical companies, leading cancer centers and academic groups to develop companion diagnostic tests in the area of oncology.
For more information on Agendia or the MammaPrint and BluePrint tests, you can visit Agendia’s patient site at www.KnowYourBreastCancer.com or the corporate site at www.agendia.com.
Follow Agendia, Inc. on Facebook, Twitter, or LinkedIn to keep up-to-date with the latest news.
Media Contacts:
Instinctif Partners
Daniel Gooch / Lynne Trowbridge
Tel: +44 (0) 20 7866 7905
Email: agendia@instinctif.com