PUBLICATIONS & ABSTRACTS: CLINICAL EVIDENCE
100s of extensive peer reviewed publications and studies utilizing MammaPrint + BluePrint have observed benefits from treatment in the following areas of clinical utility:

Scientific evidence for clinical utility.
By continually expanding and strengthening our database of proven research including 20+ years of clinical validation and 200+ research collaborations, we have gained widespread trust in the precision, accuracy and quality of our MammaPrint® and BluePrint® test suite.
Evidence
I-SPY2: ImPrint immune signature identifies ESBC patients who benefit from PD1 checkpoint inhibition
ASCO 2022, Poster #514 Authors: Kuilman et al.
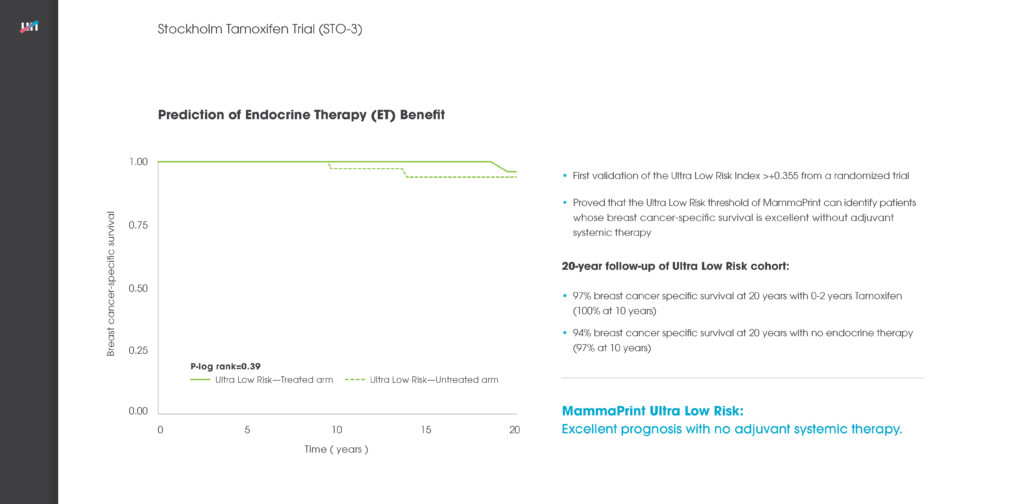
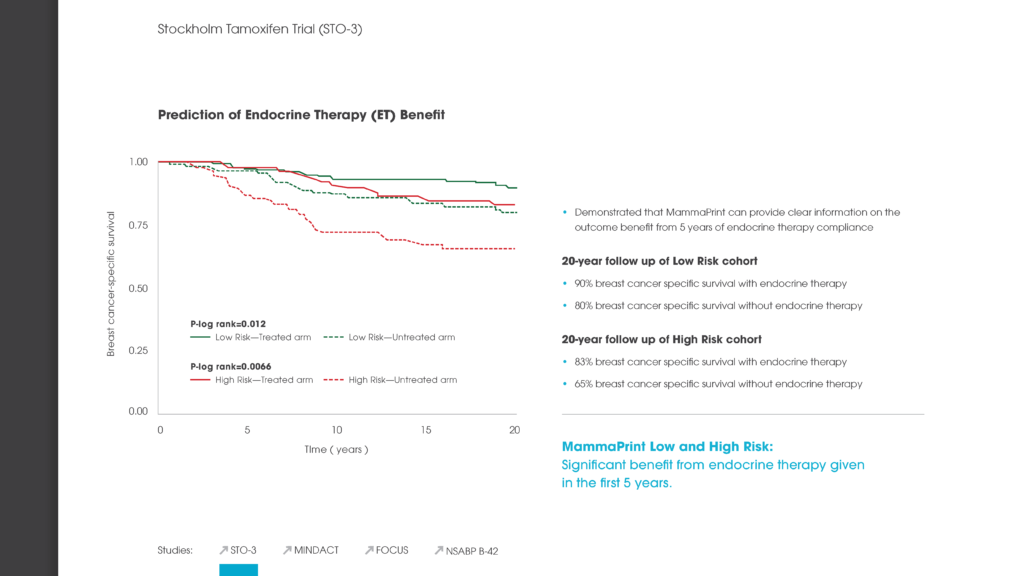
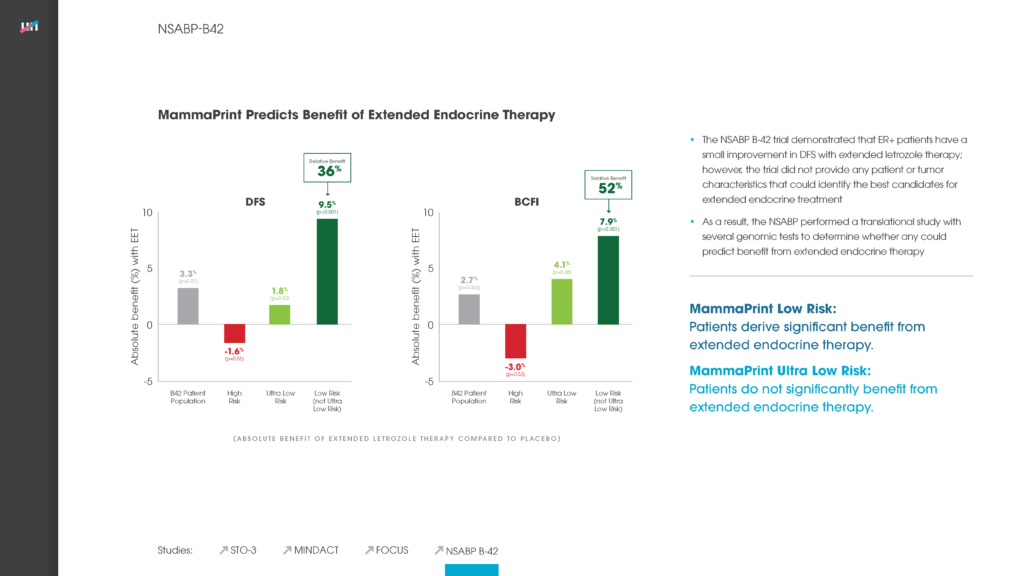
Read MoreIKA Trial: MammaPrint UltraLow & Limited Systemic Endocrine Overtreatment
IKA Trial, Breast Cancer Res Treat, 2022; 194(2): 265-278 Authors: Opdam, et al.
Read MoreBudget impact analysis for the Health Care Package by using MammaPrint in Belgium
Publication: ESMO BREAST CANCER CONGRESS 2022 Authors De Lameillieure K (1), Van der Meijden C (1), Klinkhamer J (1) , Cusumano P (2). 1- Medical & Clinical Affairs, Agendia NV., Amsterdam, Netherlands, 2 - Breast Clinic, Centre Hospitalier Chrétien, Liège, Belgium Background & Objective Gene expression profiling (GEP) tests, Read More
Equivalence of NGS-based MammaPrint 70-gene signature risk of recurrence and BluePrint 80-gene signature of molecular subtyping tests to the centralized microarray tests
Publication: ESMO BREAST CANCER CONGRESS 2022 Authors Esther Schuler(1), Sahra Uygun(2), Lorenza Mittempergher(3), Darina Pronin(3), Sammy Mee(2), Simon Bao(2), Tyson Cavness(2), Anke Witteveen(3), and Annuska Glas(3) 1. Zotz|Klimas, MVZ Düsseldorf-Centrum GbR, Germany 2. Agendia Inc., Irvine, CA 3. Agendia NV., Amsterdam, Netherlands Background The MammaPrint® 70-gene signature (70GS) risk Read More
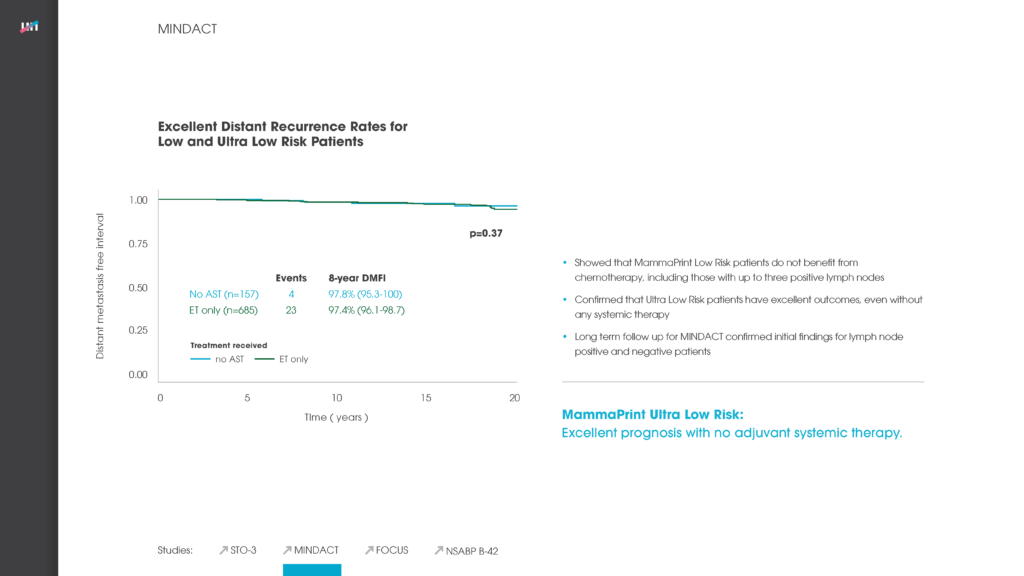
MINDACT: Outcomes of any adjuvant systemic treatment in ESBC
Annals of Oncology, Volume 33, Issue 3, March 2022, Pages 310-320 Authors: Lopes Cardozo et al.
Read MoreMINDACT Trial: Outcome of Patients with UltraLow Risk, JCO 2022
Journal of Clinical Oncology, Volume 40, Number 12, January 21, 2022 Authors: Audeh et al.
Read MoreDurvalumab + olaparib + paclitaxel in High-risk HER2-negative ESBC: I-SPY2 trial results
I-SPY2 Trial, Cancer Cell 39, 989–998, 2021 Authors: Pusztai et al.
Read More