CLINICAL DATA
20+ years of clinical validation.
200 research collaborations. 100s of publications.

Supported by a strong body of research.
The clinical utility of Agendia’s tests has been demonstrated time and again through the results of extensive clinical trials and long-standing academic and biopharma partnerships.
NBRST Trial (Neoadjuvant Breast Registry Symphony Trial)
Pre-Operative Treatment Planning
Overview
The NBRST trial (NCT01479101) was designed to demonstrate the utility of MammaPrint and BluePrint in the pre-operative treatment setting. All patients had to undergo neoadjuvant endocrine therapy or neoadjuvant chemotherapy treatment and were followed for 5 years to provide both up-front response data and longer term outcomes.
Findings
Study results showed a broad spectrum of utility for MammaPrint and BluePrint in the pre-operative setting. First both MammaPrint and BluePrint accurately predicted likelihood of pCR to neoadjuvant treatment. Further BluePrint consistently reclassified patients from their pathologic subtype into a different molecular subtype. With longer term follow up NBRST demonstrated that MammaPrint and BluePrint stratify patients into different subgroups with significantly outcomes.
In 23% of cases, tumors were reclassified from their clinical subtype to a different molecular subtype.4,5
Pathologically ER+ BluePrint Basal-Type tumors had a 34% pCR rate to neoadjuvant chemotherapy (compared to 6% in BluePrint Luminal B-Type).4,5
Luminal A-Type patients are excellent candidates for neoadjuvant endocrine therapy- they had a 52% objective response rate in the NBRST trial.4,5
MINDACT Trial (Microarray In Node-negative and 1-3 node-positive Disease may Avoid ChemoTherapy)
Adjuvant Chemotherapy Planning
Overview
MINDACT was an independent, phase III, prospective, randomized clinical trial sponsored by the European Organization for Research and Treatment of Cancer (EORTC-10041/BIG3-04). The goal of the study was to determine whether the MammaPrint test could be used to safely de-escalate clinical high risk patients with early stage breast cancer from chemotherapy treatment without compromising their outcomes.
Findings
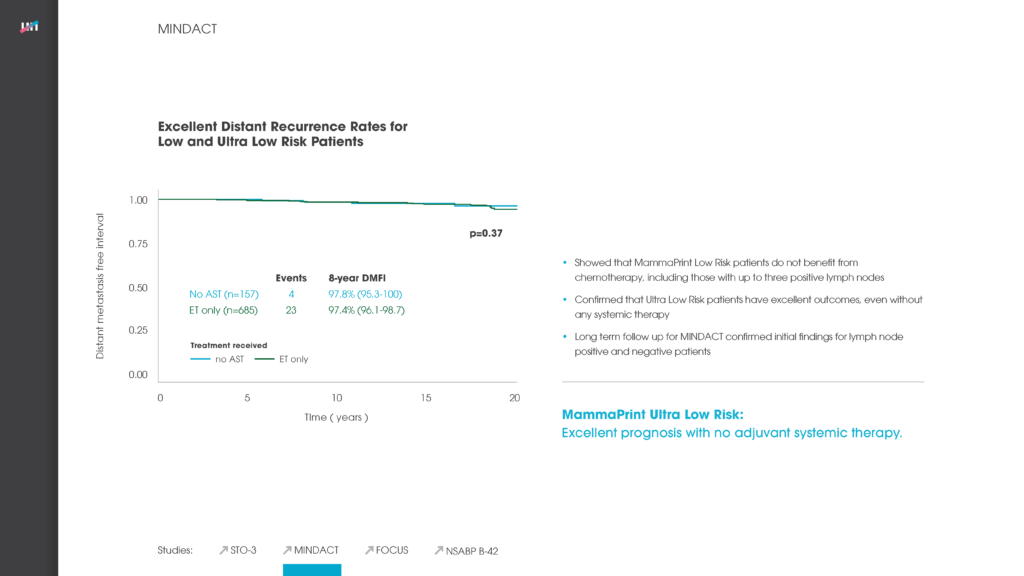
The initial data were published in the New England Journal of Medicine in 2016 and demonstrated that clinically high risk, MammaPrint Low Risk patients could avoid chemotherapy without compromising their outcomes.1 Long-term data, with median follow-up approaching 9 years, were presented at ASCO 2020 confirming and expanding upon the 2016 findings.
Nearly 50% of patients who would have received adjuvant chemotherapy were spared from that treatment with no impact on their outcomes.1
95% of clinically high risk patients, with up to 3 positive lymph nodes, who were MammaPrint Low Risk were free of distant metastases at 5 years without chemotherapy.2
Clinically high risk women over 50 with a MammaPrint Low Risk result can pursue less aggressive treatment – They had a 90% DMFS at 8 years, with or without chemotherapy.2
Patients with an Ultra Low Risk* result have excellent outcomes with no adjuvant chemotherapy or endocrine therapy – they had a 98% DMFI at 8 years.3
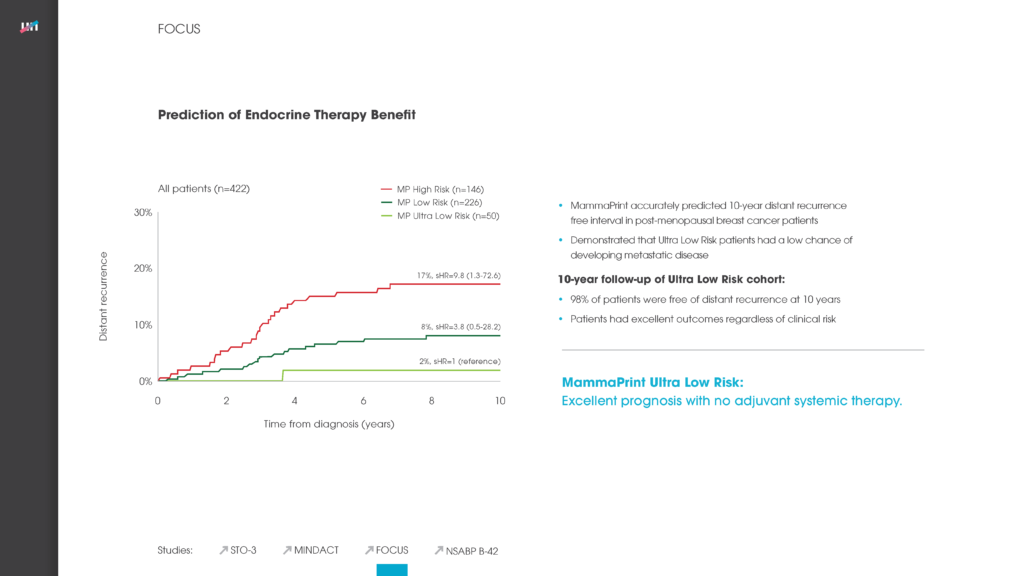
Stockholm Tamoxifen Trial (STO-3)
Finding Breast Cancers with Ultra Low Risk of Recurrence
Overview
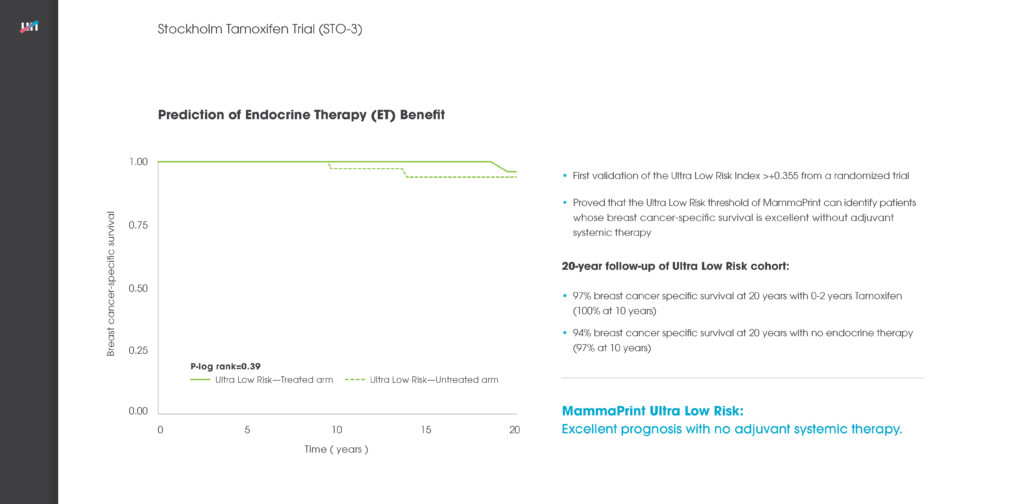
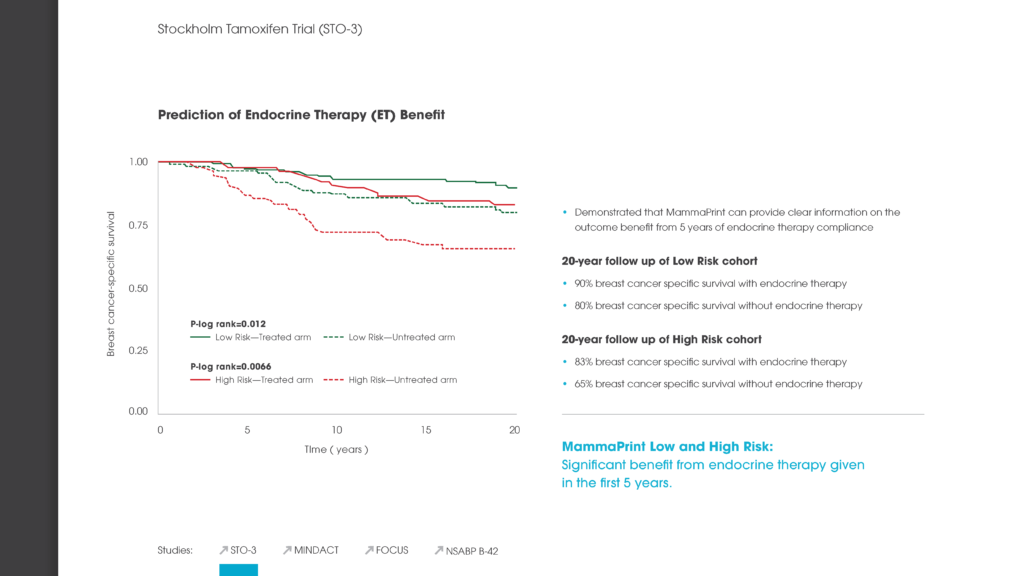
The intent of the of the Stockholm Tamoxifen Trial (STO-3) was to evaluate the efficacy of tamoxifen as an adjuvant treatment for post-menopausal women. In the Esserman et al publication in JAMA Oncology, MammaPrint was used to analyze patient samples with 20-year follow-up data from this study to determine whether the test could be used to identify extremely indolent cancers that did not need full-course endocrine therapy.
Findings
This analysis demonstrated that MammaPrint could accurately identify a subgroup of patients with exceedingly low risk of cancer recurrence 20 years after diagnosis. There was no statistically significant difference in outcomes between patients who did not receive endocrine therapy and those who received either a 2-year or a standard 5-year course of endocrine treatment.
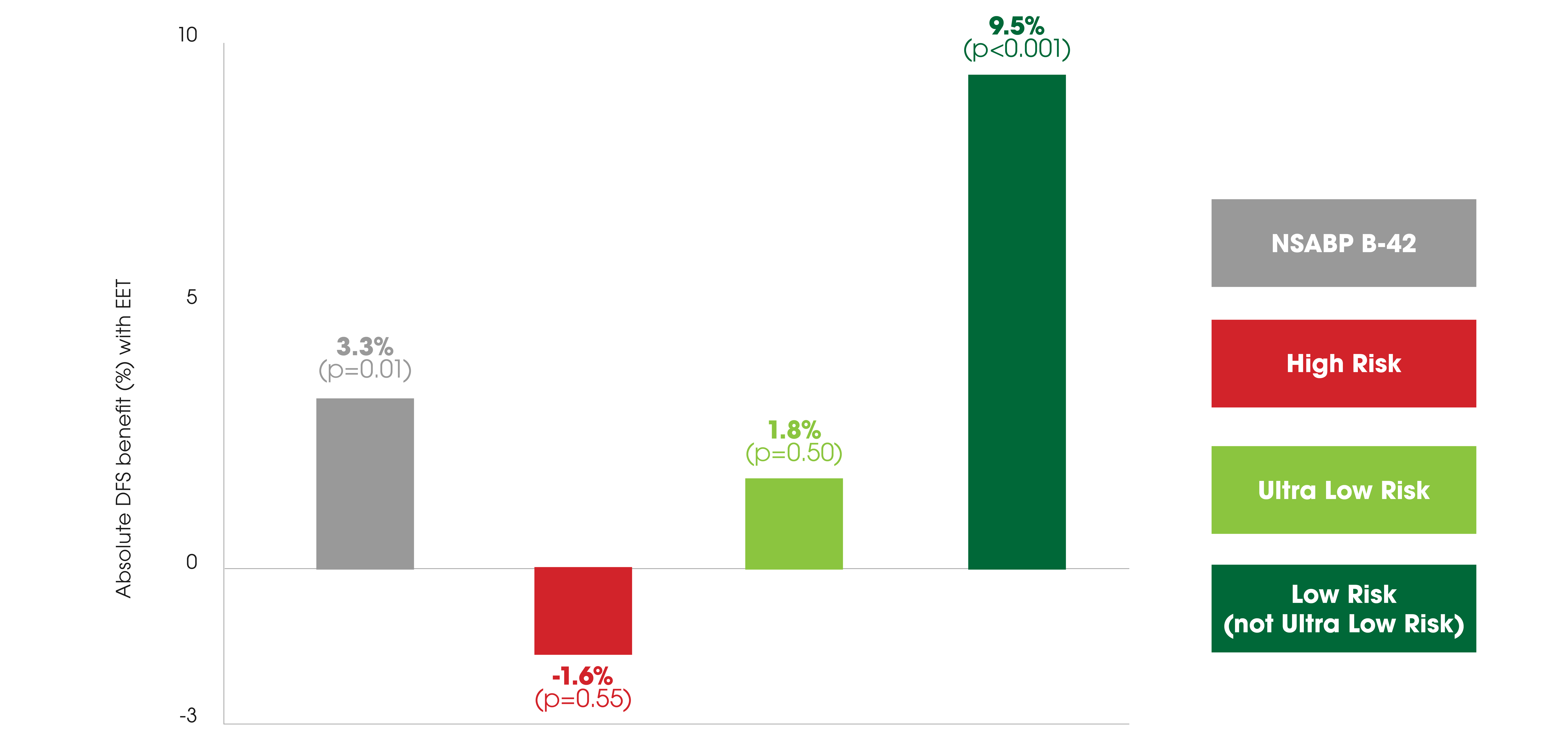
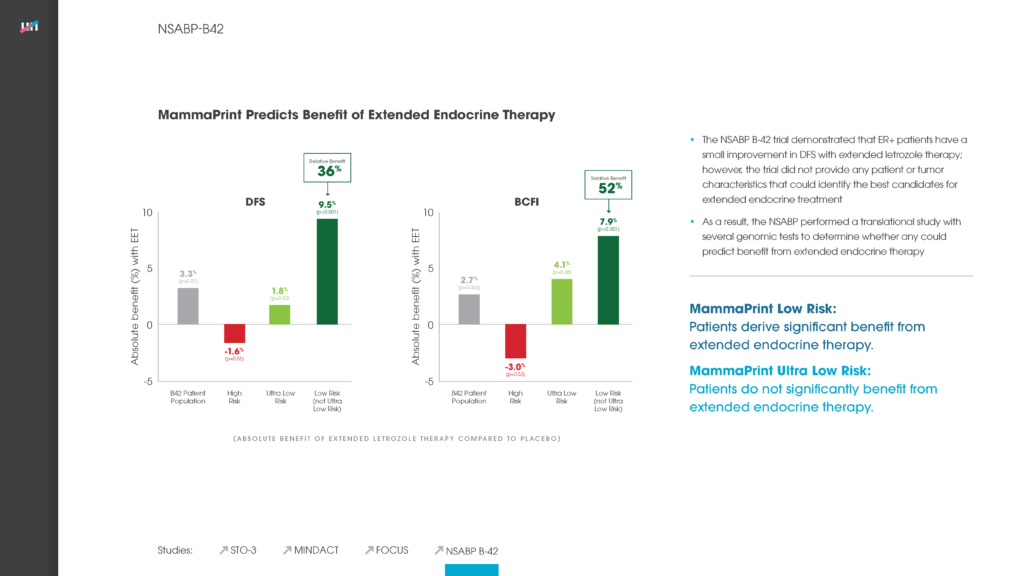
NSABP B-42
Extended Endocrine Therapy Planning
Overview
The NSABP B-42 trial demonstrated that ER+ patients have a small improvement in disease free survival (DFS) with extended letrozole therapy; however, the trial did not provide any patient or tumor characteristics that could identify the best candidates for extended endocrine treatment. As a result, the NSABP performed a translational study with several genomic tests to determine whether any could predict benefit from extended endocrine therapy.
Findings
The translational analysis demonstrated that MammaPrint could accurately predict the likelihood of benefit from extended endocrine therapy in women with early-stage breast cancer. Low Risk patients have significantly better outcomes with an additional 5 years of hormone therapy, while High and Ultra Low Risk patients do not benefit from extended endocrine treatment.
IMPACt Trial
MammaPrint and BluePrint Influence Treatment Planning
Overview
The IMPACt trial was a decision impact study intended to evaluate how MammaPrint and BluePrint results were used by physicians and whether the tests would increase or decrease physician confidence in their treatment plans.
Findings
Study results showed that MammaPrint and BluePrint results were consistently used to inform treatment planning. When clinical risk and genomic risk were discordant, physicians and their patients more often aligned their decisions with genomic results. Importantly, the study results also demonstrated that MammaPrint and BluePrint results increased physician confidence in their treatment plans in most cases.
In 40% of cases, BluePrint reclassified tumors from their pathologic subtype into a different molecular subtype.6
There was a 60% reduction in the use of chemotherapy for clinical high risk, MammaPrint Low Risk patients.6
In 72% of cases, physicians and their patients had increased confidence in their treatment plans when using MammaPrint and BluePrint.6
The science is just really good. And Agendia uses it in the best possible way to get the best possible result.
— Dr. V.K. Gadi, MD, Medical Oncologist