ABOUT US
A global leader in innovative genomic
technology and diagnostic tests.

What’s new at Agendia?
Get the latest updates on our company and products, as well as new developments in genomic science.
News
In the News
News
Media Releases
Updated NCCN® Guidelines Recognize MammaPrint® UltraLow Risk Result, Highlighting its Clinical Utility for Women with Early-Stage Breast Cancer Who Can Safely Forgo Toxic Treatments with Excellent Survival Rates
Includes level one evidence that MammaPrint can help prevent unnecessary chemotherapy¹ and endocrine therapy² Confirms Agendia’s unique ability to identify tumors that have a very low risk of distant metastasis which can have implications for Read More
Agendia Level 1B Evidence Shows MammaPrint is the First and Only FDA-cleared Gene Expression Profiling Test to Predict Benefit from Extended Endocrine Therapy in Early-Stage Breast Cancer Patients
Late-breaking abstract at the 2022 San Antonio Breast Cancer Symposium (SABCS) will illustrate MammaPrint’s ability to identify strongest candidates for extended endocrine therapy among HR+HER2- post-menopausal women December 08, 2022 09:00 AM Eastern Standard Time Read More
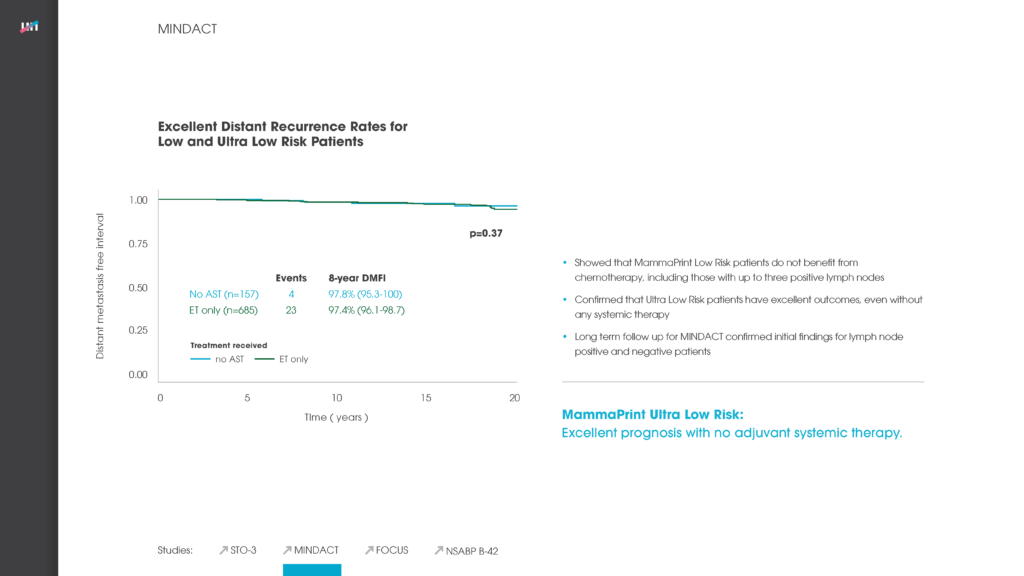
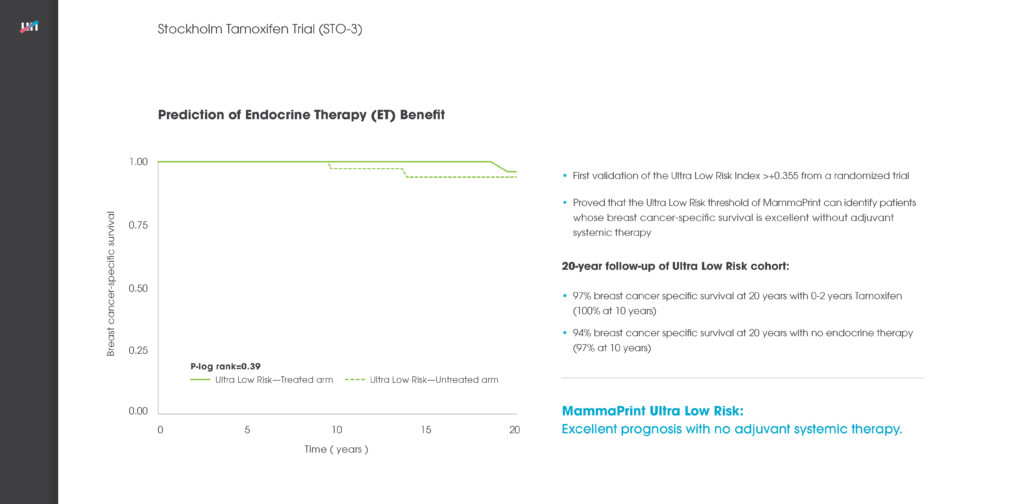
Level One Evidence Proves Premenopausal Patients within the Ultra-Low Subset of Agendia’s MammaPrint Low Risk Result Can Safely Forgo Chemotherapy
MammaPrint is the only FDA-cleared gene expression test to identify early breast cancer tumors with an Ultra-Low risk that can safely avoid chemotherapy, with a 99% breast cancer specific survival (BCSS) and 97% distant metastasis Read More
Agendia Spotlights the Future of Personalized Breast Cancer Care at 2022 San Antonio Breast Cancer Symposium
Demonstrates the ongoing impact of its 10,000-patient FLEX trial to fuel breast cancer research and produce practice-changing results All six presentations will emphasize how MammaPrint® and BluePrint® empower personalized, proactive treatment decisions with test results Read More