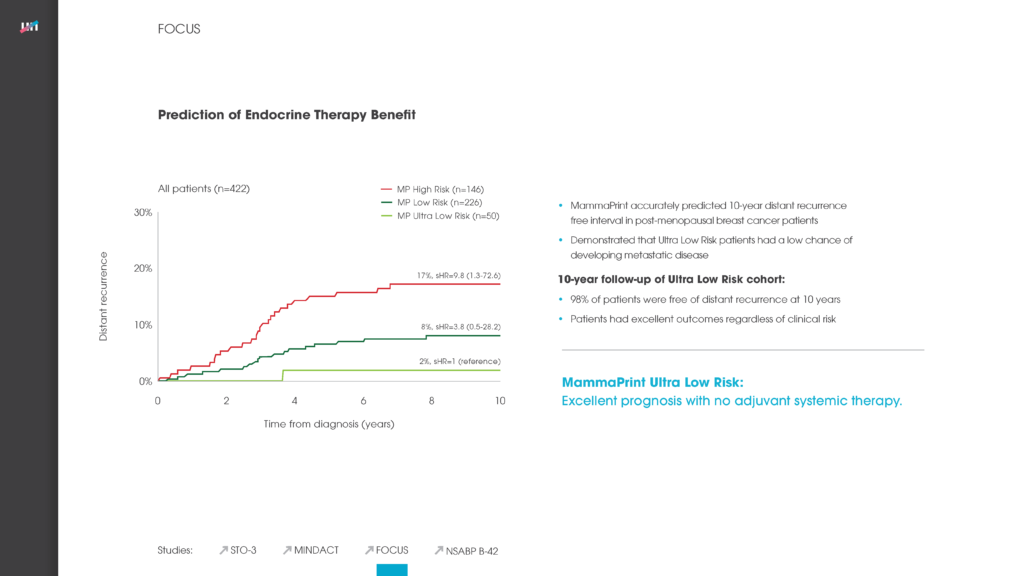
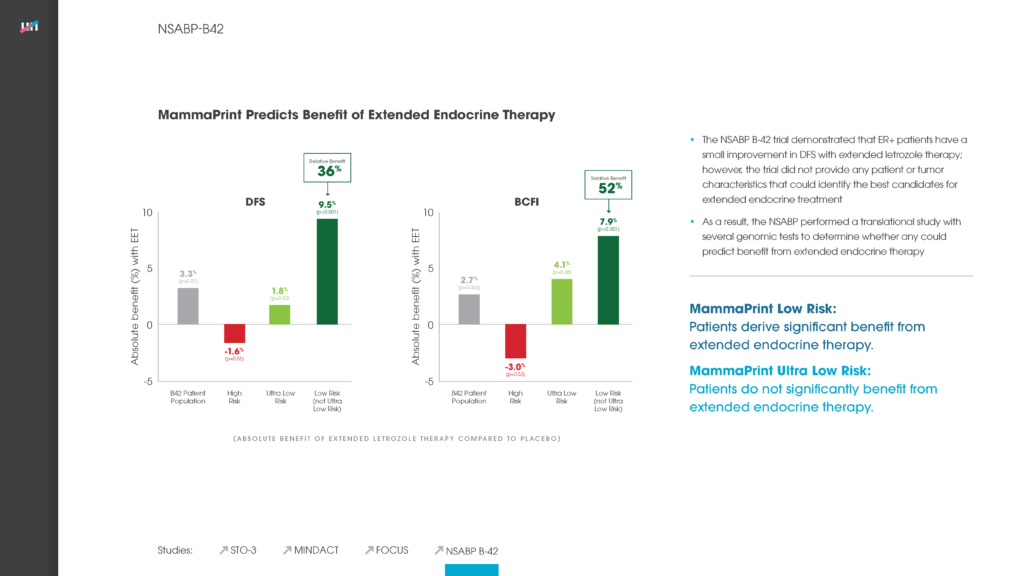
- Long-term follow-up of early-stage breast cancer patients shows significant difference in how gene expression-based tests classify patients for adjuvant chemotherapy treatment, finds MammaPrint® the best predictor of distant cancer recurrence
- MINT trial confirms stratification of patients classified by MammaPrint as High Risk into two sub-categories to help predict benefit of chemotherapy
- MINDACT sub-study compared molecular subtyping using BluePrint with pathological classification, suggests that molecular subtyping with BluePrint is better correlated with outcome
IRVINE, CA and AMSTERDAM, 14 December 2016 – Agendia, Inc., a world leader in personalized medicine and molecular cancer diagnostics, highlights new data on its gold-standard MammaPrint 70-Gene Breast Cancer Recurrence Assay and BluePrint 80-Gene Molecular Subtyping Assay, from three studies presented at the San Antonio Breast Cancer Symposium last week.
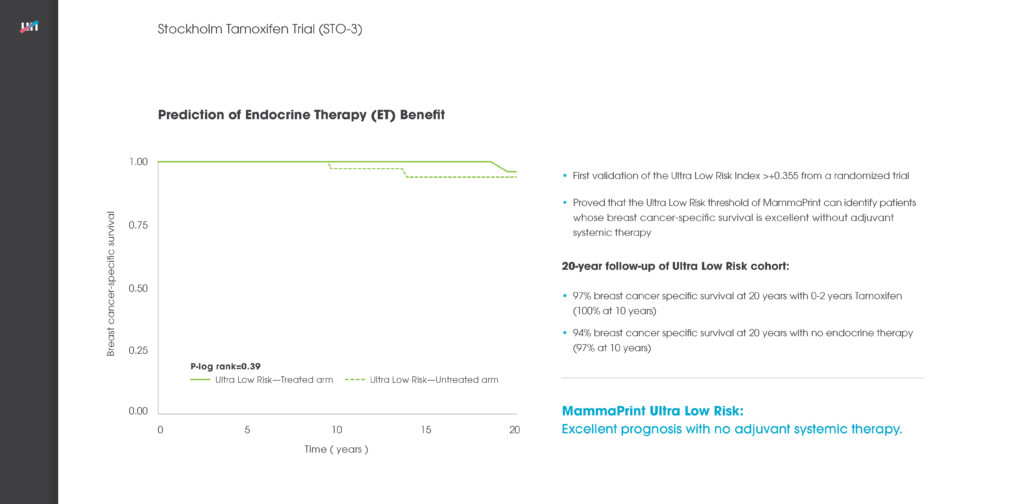
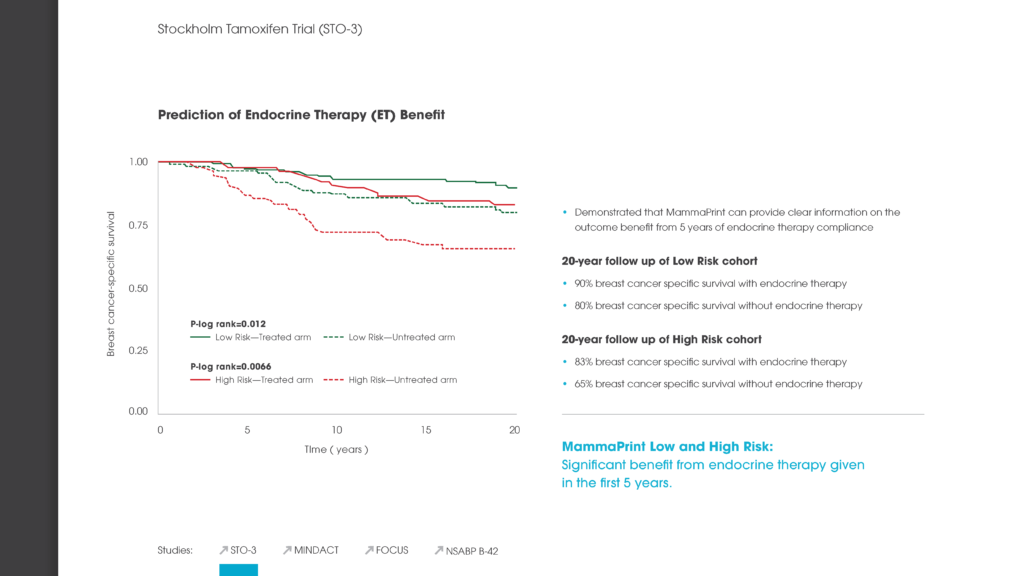
Researchers from the University of South Florida Morsani College of Medicine reported long-term follow-up data observing 148 patients who were tested with MammaPrint, Genomic Health’s Oncotype DX® and Clarient’s Mammostrat®. The study concluded that the different gene expression-based breast cancer recurrence tests showed discordant results, with only MammaPrint providing a definitive low or high risk outcome. MammaPrint also correctly reassigned a low or high risk result to intermediate Oncotype DX risk scores. The Agendia test was also the best predictor of distant cancer recurrence on identifying 85% of the 13 patients who either died or suffered a distant metastasis as high risk, compared to 54% for Oncotype DX and 62% for Mammostrat.1
The Multi Institutional Neo Adjuvant Therapy MammaPrint Project (MINT) trial demonstrated the predictive value of MammaPrint in further stratifying 183 patients classified as at high risk of their cancer recurring into High1 or High2 risk classes. Coupled with BluePrint, the study found that these MammaPrint high risk sub-groups together could help predict the benefit of chemotherapy.2
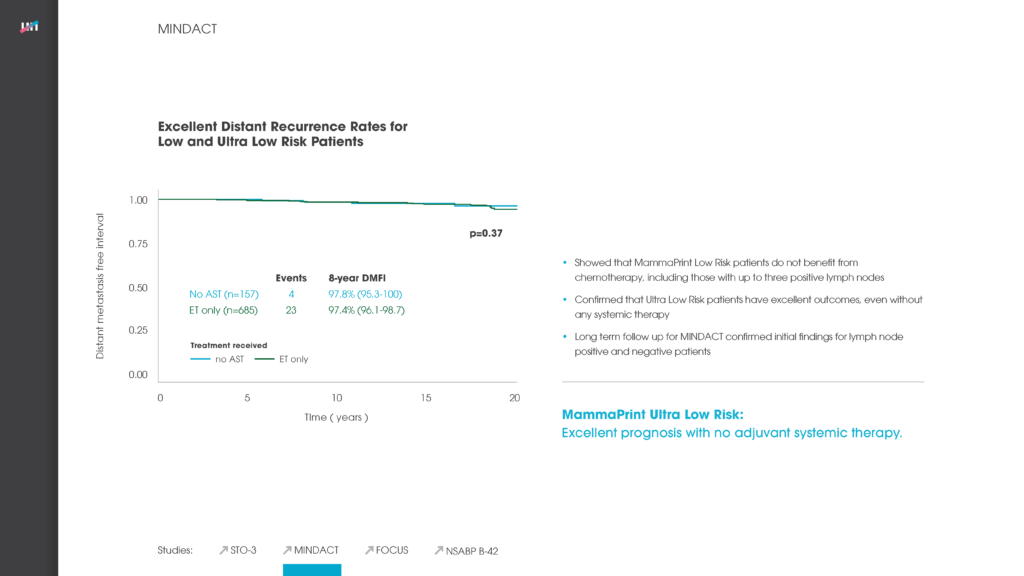
The third presentation, a sub-study of the international, prospective, phase III MINDACT trial, published in August 2016, compared molecular subtyping using BluePrint to pathological classification. Molecular subgroups within early-stage breast cancer (such as Luminal A, Luminal B, HER2+, Basal-like) may help to best identify patients for specific treatment approaches. A key conclusion was that molecular subtyping using BluePrint was able to restratify 16% of patients, originally classified pathologically high risk, to a low risk Luminal A-type group, with excellent survival. The study suggests that molecular subtyping by BluePrint is better correlated with outcome than pathological classification.3
The MINDACT trial, which was published in the New England Journal of Medicine, is a unique phase III prospective, randomized, controlled study that provides the highest level of clinical evidence to MammaPrint, above any other genomic assay, for making adjuvant chemotherapy decisions in early-stage breast cancer. It included almost 7,000 patients, across 112 institutions in nine different European countries.
William Audeh, MD, Chief Medical Officer at Agendia said: “As the only gene expression-based breast cancer recurrence test supported by Level 1A clinical evidence, it is very good to see the value of MammaPrint, and also BluePrint demonstrated by these new data at this important symposium. The results highlight the benefit of these tests in providing patients with early stage breast cancer, and their physicians, with the confidence to make important, informed treatment decisions. It also demonstrates the potential to further apply our technology to truly individualize the prognosis and the prediction of the best breast cancer treatment, one patient at a time.”
For more information on Agendia or the MammaPrint and BluePrint tests, you can visit Agendia’s patient site at KnowYourBreastCancer.com or the corporate site at Agendia.com.
Follow Agendia, Inc. on Facebook, Twitter, or LinkedIn to keep up-to-date with the latest news.
1Russell S, et al. Long-term follow-up of early stage breast cancer patients with results of MammaPrint, Oncotype DX, and MammoStrat, risk classification assays. Poster presented at San Antonio Breast Cancer Symposium. December 2016; San Antonio, Texas.
2Blumencranz L, et al. Mint trial yields MammaPrint High1/High2 risk classes associated with significant differences in pCR and receptor subtype. Poster presented at San Antonio Breast Cancer Symposium. December 2016; San Antonio, Texas.
3Cardoso F, et al. Can Surrogate Pathological Subtyping Replace Molecular Subtyping? Outcome Results from the MINDACT Trial. Poster presented at San Antonio Breast Cancer Symposium. December 2016; San Antonio, Texas.
About MammaPrint
MammaPrint is a FDA-cleared in vitro diagnostic test, available from FFPE sample, performed in a single laboratory, using the gene expression profile of breast cancer tissue samples to assess a patients’ risk for distant metastasis. The MammaPrint result is indicated for use by physicians as a prognostic marker only, along with other clinical-pathological factors. MammaPrint is not intended for diagnosis, or to predict or detect response to therapy, or to help select the optimal therapy for patients. Results should be taken in the context of other relevant clinical-pathological factors and standard practice of medicine.
About Agendia
Agendia is a privately held, leading molecular diagnostics company that develops and markets FFPE-based genomic diagnostic products, which help support physicians with their complex treatment decisions. Agendia’s breast cancer tests were developed using an unbiased gene selection by analyzing the complete human genome. Our offerings include the FDA-cleared MammaPrint FFPE 70-gene breast cancer recurrence assay as well as BluePrint®, a molecular subtyping assay that provides deeper insight leading to more clinically actionable breast cancer biology. These tests can help physicians assess a patient’s individual risk for metastasis – that is, which patients are more sensitive to chemo, hormonal, or combination therapy, and which patients may not require these treatments and which patients may be treated with other, less arduous and costly methods.
In addition, Agendia has a pipeline of other genomic products in development. The company collaborates with pharmaceutical companies, leading cancer centers and academic groups to develop companion diagnostic tests in the area of oncology. For more information, visit www.agendia.com.
Media Contacts:
Scott Speer (US media)
FleishmanHillard
(310) 482-4283
scott.speer@fleishman.com
Léon Melens / Jen Lewis / Dana Garbe (EU media)
Instinctif Partners
+31 6 538 16 427 / +44 20 7457 2020 / +49 30 2408304-11
agendia@instinctif.com